|
Back to Main Research page |
|
|
|
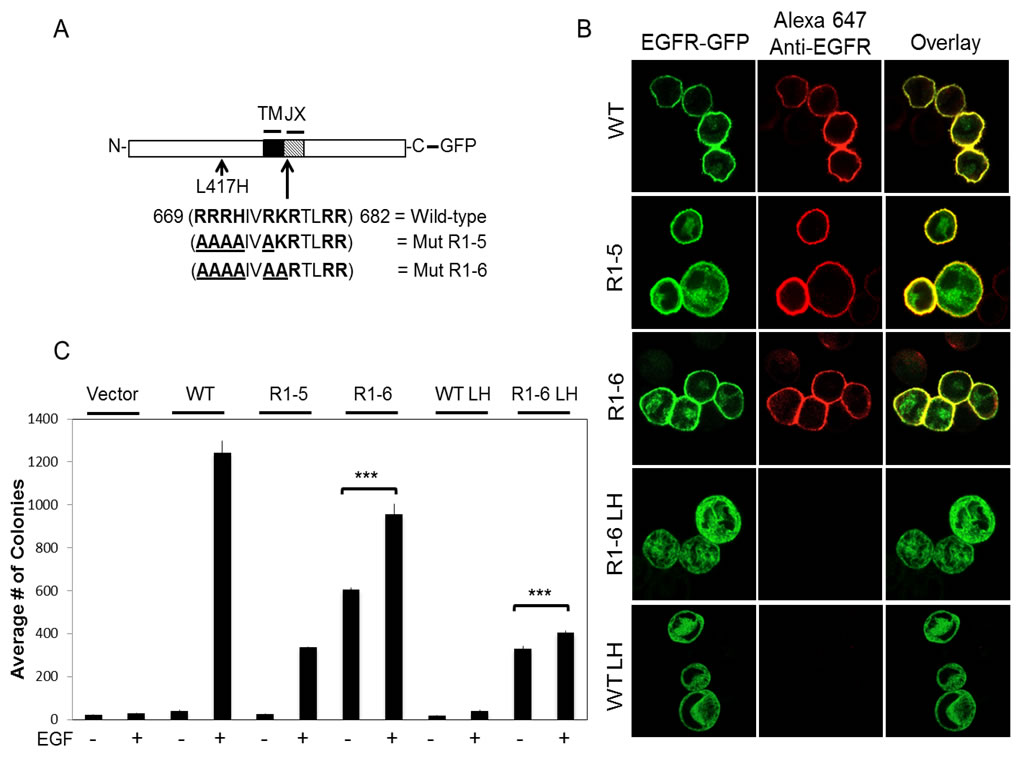 |
Ligand-independent cell transformation by an ER-retained EGF Receptor MutantReceptor tyrosine kinases (RTKs) initiate some of the largest signaling networks in cells. The ErbB family, specifically ErbB1, or the epidermal growth factor receptor (EGFR), serves as the prototypic model for RTK signaling. Conserved in the ErbB family is a cluster of basic amino acid residues in the juxtamembrane (JX) domain. We find that a single point mutation, L417H, fully retains EGFR in the ER, and charge-silencing mutagenesis within the juxtamembrane (JX) region yields a mutant (EGFR R1-6) that is constitutively active. We show that EGFR R1-6 with L417H is ER-retained, exhibits basal tyrosine phosphorylation, and transforms NIH 3T3 cells in a ligand-independent manner when ectopically expressed. This transforming activity is sensitive to inhibition of EGFR kinase and is dependent on PI3-kinase and mTOR activity. Our findings demonstrate that ER-localized EGFR mutants are capable of potent transforming activity. |
||
| (A) Wild-type and mutant EGFR-GFP constructs generated. The JX sequence in EGFR is shown, with basic amino acids in bold and mutated residues underlined. (TM = transmembrane) (B) Expression of wt and mutant EGFR-GFP constructs in suspended RBL mast cells. Transiently expressing cells were fixed and labeled with an anti-N-terminal EGFR antibody, followed by Alexa647-anti-IgG. (C) NIH 3T3 fibroblasts stably expressing vector, wt EGFR, EGFR Mut R1-5, EGFR Mut R1-6, EGFR L417H, or EGFR Mut R1-6/L417H, incubated with or without EGF (100 ng/ml), were cultured in soft agar. The data shown represent the mean ± SD for colonies per 2.25 cm2 from at least three independent experiments. ***, P<0.001.
Bryant, K.L., M.A. Antonyak, R.A. Cerione, B. Baird and D. Holowka: Mutations in the Polybasic Juxtamembrane Sequence of Both Plasma Membrane- and Endoplasmic Reticulum-localized Epidermal Growth Factor Receptors Confer Ligand-independent Cell Transformation. J. Biol. Chem. 288(48): 34930-34942 (2013). Bryant, K.L., B. Baird and D. Holowka: A novel fluorescence-based biosynthetic trafficking method provides pharmacologic evidence that PI4-kinase IIIa is important for protein trafficking from the endoplasmic reticulum to the plasma membrane. BMC Cell Biology 16: 5/1-5/12 (2015). |
|||
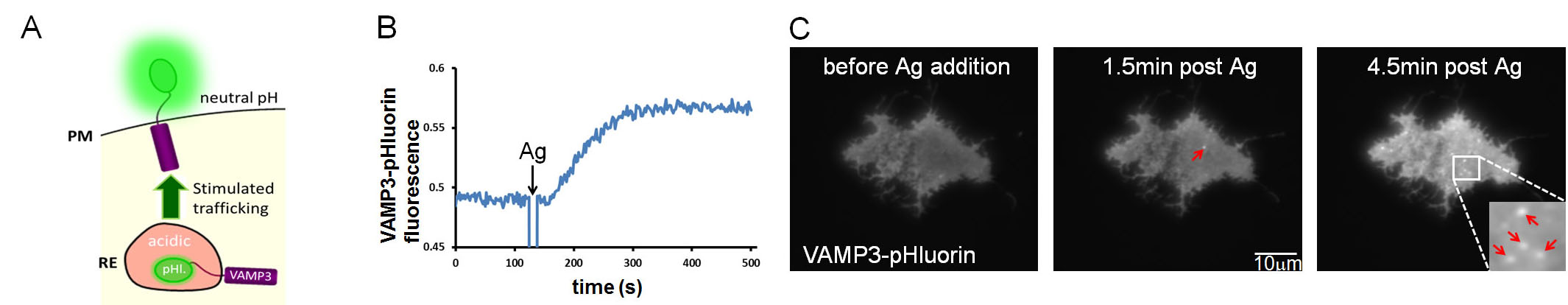
Stimulated recycling endosome exocytosis in mast cellsStimulated membrane trafficking events are a hallmark of the allergic response. These processes are triggered by multivalent antigen-mediated crosslinking of IgE bound to its high affinity Fc receptor (FceRI) on the mast cell surface. In addition to stimulating degranulation, previous work in our laboratory has demonstrated that antigen-mediated crosslinking of cell-surface IgE-FceRI also initiates the exocytosis of recycling endosome (RE)-derived membranes. My initial work has focused on the development of molecular tools to monitor RE exocytosis. A fundamental aspect of these methods is fluorophore dequenching mediated by the transition from the acidic RE lumen to the neutral pH of the extracellular space (A). These approaches include fluorimetry-based (B) and microscopy-based (C) assays that monitor VAMP3, an RE-localized SNARE protein, fused to a pH-sensitive variant of GFP (pHluorin). These methods will allow us to quantitatively assess the influence of genetic and pharmacologic perturbations on stimulated RE exocytosis (fluorimetry), as well as to examine the spatial behavior of this process (imaging). Ultimately, we seek to understand how stimulated RE exocytosis is integrated with other events that, as a whole, mediate mast cell responses to antigen. Wilson, J.D., S.A. Shelby, D. Holowka and B. Baird: Rab11 Regulates the Mast Cell Exocytic Response. Traffic 17(9): 1027-1041 (2016). |
|||
 |
|||
| Antigen-stimulated recycling endosome exocytosis monitored with VAMP3-pHluorin.(A) Depiction of VAMP3-pHl. (VAMP3 fusion with super ecliptic pHluorin): fluorescence dequenching by repositioning from the acidic environment of the recycling endosome (RE) lumen to the neutral pH of the extracellular space.(B) An example of VAMP3-pHluorin exocytosis monitored by fluorimetry. RBL-2H3 mast cells were transfected with VAMP3-pHl. and sensitized with anti-DNP IgE prior to harvest. Multivalent antigen (Ag, 100 ng/ml DNP-BSA, dinitrophenyl-bovine serum albumin) was added at the indicated time. The fluorescence increase indicates elevated exocytosis of VAMP3-pHl.-labeled membrane.(C) VAMP3-pHl. exocytosis in response to Ag monitored by TIRF (total internal reflection fluorescence) microscopy. Bright puncta (red arrows) are exocytic events. Increased plasma membrane fluorescence is also apparent. RBL-2H3 mast cells were transfected and sensitized as in B. and stimulated with 200 ng/ml Ag. | |||
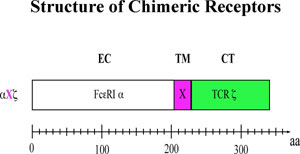
Transmembrane Sequences are Determinants of Immunoreceptor SignalingWhat are the structural features critical for signal initiation by antigen-stimulated IgE receptor FceRI? We investigated the role of the transmembrane (TM) sequence of the IgE receptor in its signaling. To look at the structural features of immunoreceptors critical for lipid raft-dependent signaling we prepared and characterized single-chain receptors that contain the essential structural features of the high affinity IgE receptor, FceRI. Our constructs contain extracellular human FceRI for IgE binding, a variable TM region, and the ITAM-containing cytoplasmic tail of the T cell receptor z subunit. We selectively evaluated expression, morphological changes, raft localization, phosphorylation, calcium response, and degranulation due to crosslinking the chimeric IgE receptors in RBL mast cells stably transfected with these chimeric receptors. |
|
||
|
Stimulation of tyrosine phosphorylation, calcium mobilization, degranulation and lipid raft association are strongly dependent on the chimeric receptor TM sequences, and these responses are highly correlated to crosslink-dependent association with detergent-resistant lipid rafts. Those with TM domains from non-raft proteins (aPz, a45z) gave very small responses. For the chimera aFz, mutation of a TM cystein abolishes robust signaling an lipid raft association. In addition, TM disulfide-mediated dimerization of azz enhances signaling. Gosse, J.A., A. Wagenknecht-Wiesner, D. Holowka and B. Baird: Transmembrane Sequences Are Determinants of Immunoreceptor Signaling. J. Immunol. 175(4): 2123-2131, 2005. |
|||