|
Back to Main Research page |
|||
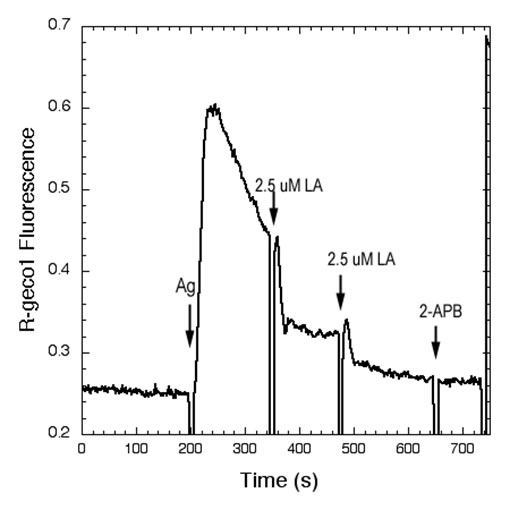
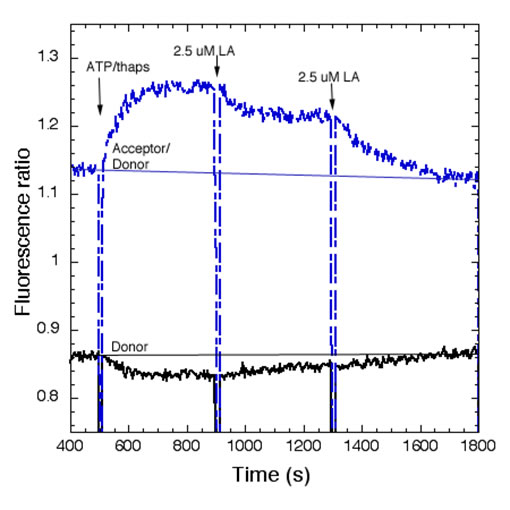
Polyunsaturated Fatty Acids Interfere with Coupling Between the ER Ca2+ Sensor, STIM1, and the Ca2+ Channel Protein, Orai1As for many other receptors that activate Ca2+ mobilization to mediate functional responses, the endoplasmic reticulum (ER) Ca2+ sensor, STIM1, and the plasma membrane (PM) Ca2+ channel, Orai1, play key roles in IgE receptor signaling in mast cells. In this process, stimulated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol 1,4,5-trisphosphate (IP3) is essential for initiation of store-operated Ca2+ entry (SOCE) and granule exocytosis. To further characterize the roles of membrane structure in receptor-activated SOCE, we are investigating the effects of free fatty acids on FceRI-activated Ca2+ mobilization. We find that addition of micromolar concentrations of C18:2 (n-6) linoleic acid (LA) rapidly inhibits FceRI-activated Ca2+ mobilization by inhibiting both antigen-stimulated release of Ca2+ from ER stores, as well as by inhibiting SOCE (Fig. 1 and data not shown). In COS7 cells we monito r thapsigargin-stimulated coupling between STIM1 and Orai1 using fluorescence resonance energy transfer (FRET) between these labeled proteins (5). We find that LA directly interferes with this association, whereas the C18 saturated fatty acid counterpart, stearic acid, does not inhibit this process or Ca2+ mobilization (Fig. 2 and data not shown). Under conditions in which Ca2+ mobilization by antigen is inhibited >90% by linoleic acid, no effects on stimulated tyrosine phosphorylation upstream of Ca2+ mobilization are detected. We conclude that STIM1-Orai1 coupling is particularly sensitive to perturbation by unsaturated fatty acids, and we hypothesize that these may directly alter ER membrane structure. Holowka, D., M.K. Korzeniowski, K.L. Bryant and B. Baird: Polyunsaturated fatty acids inhibit stimulated coupling between the ER Ca2+ sensor STIM1 and the Ca2+ channel protein Orai1 in a process that correlates with inhibition of stimulated STIM1 oligomerization. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 1841(8): 1210-1216 (2014). Holowka, D., K. Thanapuasuwan and B. Baird: Short chain ceramides disrupt immunoreceptor signaling by inhibiting segregation of Lo from Ld Plasma membrane components. Biology Open 7(9): 034702-1-9 (2018). |
|||
 |
 |
||
| Fig 1.Linoleic acid (LA) rapidly inhibits Ag-stimulated Ca2+ mobilization. Ca2+ mobilization monitored by the genetically encoded indicator R-geco1 is stimulated by an optimal dose of multivalent antigen (Ag; 0.2 mg/ml DNP15-BSA) for anti-dinitrophenyl (DNP) IgE-sensitized RBL mast cells, and the sustained phase of this response is rapidly inhibited by two successive additions of 2.5 mM LA. The SOCE inhibitor 2-APB does not further inhibit this response. | Fig 2. Stimulated FRET between AcGFP-Orai1 and STIM1-mRFP in COS7 cells is reversed by linoleic acid (LA). Addition of LA subsequent to stimulation of coupling between AcGFP-Orai1 and STIM-mRFP by ATP/thapsi-gargin interferes with this association. FRET was monitored simultaneously as normalized donor quenching (black traces) and as the ratio of sensitized acceptor fluorescence to donor emission (blue traces). | ||
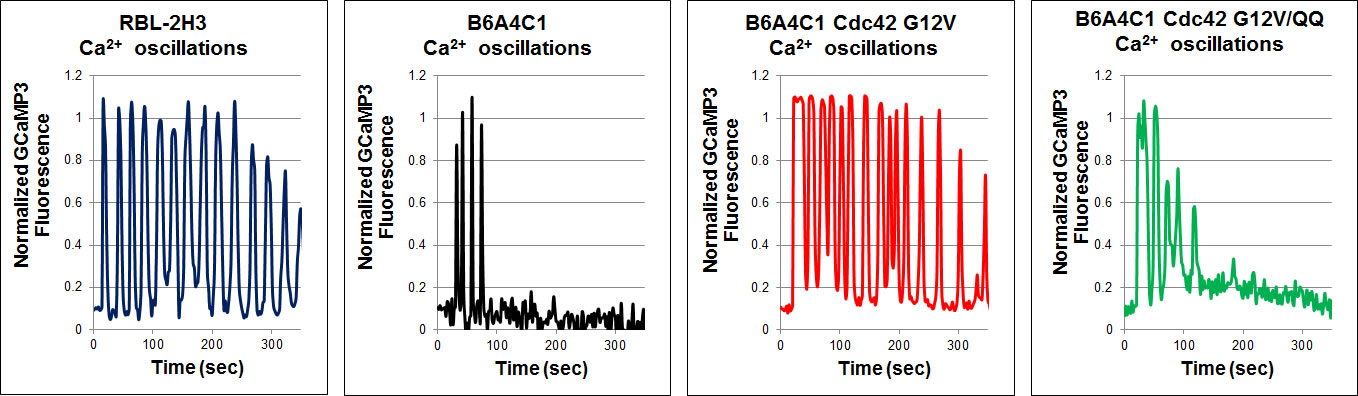
Reconstitution of sustained FceRI-mediated Ca2+ oscillations in mutant RBL mast cells by the Rho family GTPase Cdc42Antigen stimulation of mast cells via FceRI, the high-affinity receptor for IgE, triggers a signaling cascade that requires Ca2+ mobilization for exocytosis of secretory granules during an allergic response. Mutant RBL-2H3 cells, designated B6A4C1, are defective in FceRI-mediated Ca2+ mobilization and degranulation. As part of their reduced Ca2+ responses, B6A4C1 exhibit attenuated Ca2+ oscillations compared to wild type RBL-2H3 cells, and it is well established that sustained Ca2+ oscillations are necessary for effective degranulation. Our laboratory previously demonstrated that the constitutively active small GTPase, Cdc42 G12V, has the capacity to reconstitute Ca2+ mobilization and consequent exocytosis in these mutant B6A4C1 cells.In reconstituting antigen stimulated Ca2+ release from stores and store operated Ca2+ entry, we now find that Cdc42 G12V restores Ca2+ oscillations in B6A4C1 cells to wild type RBL-2H3 levels. A recent study showed that a C-terminal di-arginine motif of Cdc42 is essential for the binding of Cdc42 to PIP2-containing membranes and for 3T3 fibroblast transformation (Johnson et al, JBC 2012). We find that this motif is necessary for Cdc42 to reconstitute antigen mediated Ca2+ responses, including sustained oscillations, in the mutant RBL-2H3 cells. These finding have begun to give us new insights into potential mechanisms by which Cdc42 may be regulating Ca2+ responses in RBL-2H3 cells. Wilkes, M.M., J.D. Wilson, B. Baird and D. Holowka: Activation of Cdc42 is necessary for sustained oscillations of Ca2+ and PIP2 stimulated by antigen in RBL mast cells. Biology Open 3(8): 700-710 (2014). |
|||
 |
|||
| Mutant B6A4C1 cells exhibit attenuated Ca2+ oscillations compared to wild type RBL-2H3 cells, whereas expression of active Cdc42 G12V reconstitutes more sustained oscillations in these cells. Mutation of the C-terminal di-arginine motif to di-glutamine (Cdc42 G12V/QQ) prevents this reconstitution. Antigen was added at time zero for all graphs. | |||
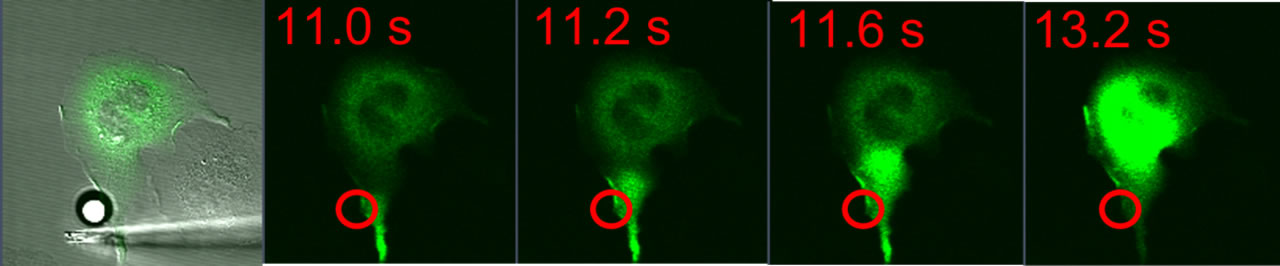
Antigen-Mediated Spatiotemporal Ca2+ Signaling and Degranulation in RBL-2H3 Cells by Stimulation with Antigen-Coated BeadsWe recently showed that antigen-stimulated Ca2+ mobilization via IgE receptors in RBL-2H3 mast cells usually initiates as a fast Ca2+ wave that begins at the tip of an extended protrusion, and is followed by global Ca2+ oscillations (Cohen et al. J. Immunol. 183:6478). The complex spatial and temporal regulation of Ca2+ responses in mast cells influences downstream responses including degranulation and transcriptional regulation of the synthesis of inflammatory mediators. To further investigate the spatial aspects of IgE signaling, we are locally stimulating RBL cells with 10 μm antigen-coated polystyrene beads. Cells transfected with the genetically-encoded Ca2+ indicator GCaMP3 respond to bead stimulation with Ca2+ waves initiating near bead placement, followed by both global oscillations and localized Ca2+ transients near the bead.Fluorescently-tagged PKC localizes to the plasma membrane near the site of stimulation, suggesting localized activation. Granule exocytosis, observed by fluorescence imaging of FITC-dextran release (Cohen et al. J Cell Sci , 125(Pt 12):2986-94), is limited when compared with stimulation by soluble antigen, and this exocytosis is localized to the site of stimulation by the bead. The granule exocytosis response is enhanced by inhibition of actin polymerization, which also delocalizes this response. Future experiments will investigate the role of actin cycling in the localization of these cellular responses. |
|||
 |
|||
| RBL-2H3 cell transfected with GCaMP3 and stimulated with a 10 μm antigen-coated bead (white, left panel). Green fluorescence images show a calcium wave originating in the cellular protrusion near the site of bead contact (red circle) and traversing the length of the cell. This calcium wave is followed by localized calcium oscillations near the site of the bead (not shown). | |||
Studies of the Molecular Conformation of STIM1Store operated Ca2+ entry (SOCE) is an important mechanism for signaling in hematopoietic cells to maintain Ca2+ homeostasis. Understanding the molecular orchestration of SOCE is a current goal of many laboratories.This process depends on oligomerization of the luminal ER Ca2+ sensor, STIM1, and co-clustering with the Ca2+ channel protein, Orai1, at endoplasmic reticulum (ER)-plasma membrane (PM) junctions. Activation of STIM1 occurs when the ER luminal Ca2+ concentration decreases in response to PIP2 hydrolysis at the PM. STIM1 oligomerization is followed by its translocation to ER-PM junctions where STIM1 binds and activates oligomeric Orai1, a process visible as puncta formation. We are characterizing STIM1 oligomerization dynamics at the nanoscale using fluorescent fusion proteins and fluorescence resonance energy transfer (FRET) to monitor molecular associations. Korzeniowski, M.K., B. Baird and D. Holowka: STIM1 activation is regulated by a 14 amino acid sequence adjacent to the CRAC activation domain. AIMS Biophysics 3(1): 99-118 (2016). Korzeniowski, M.K., E. Wisniewski, B. Baird, D.A. Holowka and T. Balla: Molecular anatomy of the early events in STIM1 activation – oligomerization or conformational change? J. Cell Sci. 130(17): 2821-2832 (2017). |
|||
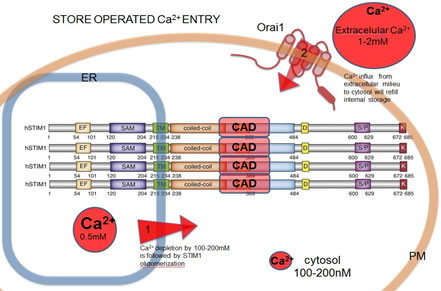 |
Activation of SOCE involves co-clustering of the ER Ca2+ sensor STIM1 and the PM Ca2+ channel protein Orai1. ER Ca2+ store depletion triggers STIM1 oligomerization known to be the initial step in SOCE. Activated Stim1 and Orai1 are found in PM-ER junctions, where the distance between membranes is ~20nm and they form micron-scale puncta. Ca2+ influx via SOCE triggers signaling cascades pivotal for maintaining cell integrity and function. The capacity of STIM1 to form oligomers is a key to the mechanism of SOCE activation. Still unknown is the molecular orchestration of conformational changes leading to Ca2+ influx through Orai1 channels. | ||
| Current data suggest that STIM1 is minimally dimeric due to interactions between cytoplasmic CRAC activation domains (CAD). We previously identified a basic sequence inside CAD responsible for activation of Orai1,and an acidic glutamate-rich region near CAD that appears to regulate a conformation change in STIM 1 upon activation. In recent studies we developed a fluorimetry-based FRET method to monitor STIM1 oligomerization changes at both the luminal (N-terminal) side and the cytoplasmic (C-terminal) side of STIM1, and we are monitoring these interactions and their alterations due to mutations in STIM1. | |||
 |
 |
||
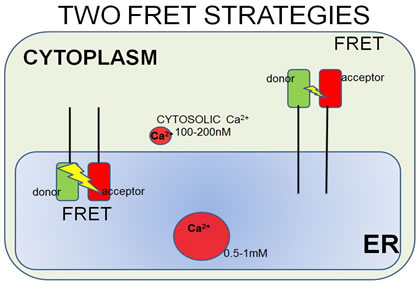
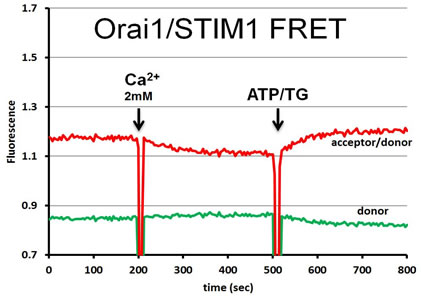
| FRET strategies. STIM1 is a type I transmembrane ER protein that forms oligomers during Ca2+ depletion from the ER lumen. We hypothesize that both luminal and cytosoplasmic segments of STIM1 change their conformations during Ca2+ store depletion and Orai1 coupling. FRET experiments were designed to follow changes in STIM1-STIM1 proximity during Ca2+ store refilling (Ca2+ addition) and during SOCE activation by ATP + thapsigargin. STIM1 was either N-terminally tagged with YFP and mRFP, co-expressed to monitor luminal changes in one FRET strategy (left side), or it was C-terminally-tagged with AcGFP and mApple, co-expressed to monitor cytoplasmic changes in another strategy (right side). | In a related type of experiment, STIM1–Orai1 association at the nanometer scale can be monitored by steady-state fluorimetry measurements of FRET between AcGFP-Orai1 and STIM1-mApple. | ||
