Back to Main Research page
Alpha synuclein binding to mitochondria increases after toxin (CCCP) stress
Alpha synuclein (a-syn) is a major component of Lewy bodies, a neuropathology observed in Parkinson’s disease (PD) and other related neurodegenerative disorders. Much of our knowledge concerning a-syn stems from in-vitro studies focused on the aggregation process and on membrane binding properties. Despite the development of cell and animal models, the functional consequence of a-syn’s structural interactions within cells remain poorly defined.
We use multiple mammalian cell models to measure and analyze effects of a-syn on specific cell processes. In initial studies we investigated phenotypes of a-syn overexpression in RBL and PC-12 cells by monitoring stimulated exocytosis of recycling endosomes (REs) as a proxy for synaptic vesicles that are similar in size. We demonstrated that lower expression levels of a-syn inhibit exocytosis of REs while higher expression levels lead to some enhancement. Using NMR spectroscopy in collaborative studies, we demonstrated that membrane binding properties of a-syn variants correlate with the functional outcomes. In particular, perturbation in N-terminal helices 1 and 2 by proline mutations, specifically affects both binding affinity and participation in RE exocytosis. On the other hand, C-terminal truncation and PD-related mutations behave similarly to wt a-syn at low expression levels in the exocytosis assay, indicating some regions of a-syn not involved in this mechanism.
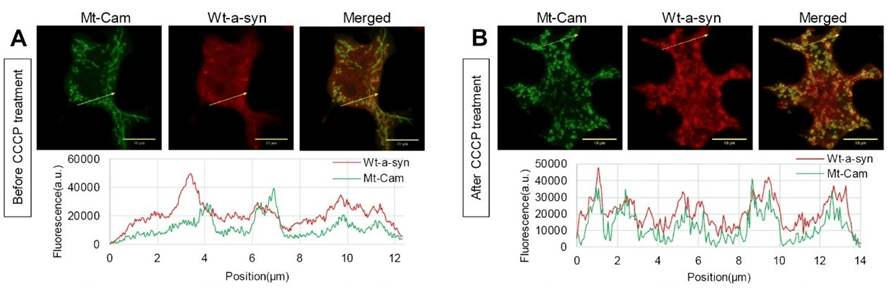
In other experiments, we found that wt a-syn binds weakly to mitochondria, but not helix 1/2 proline mutants. Interestingly, wt a-syn binding becomes considerably stronger upon mitochondrial stress (Figure). We are now finding that expression of a-syn enhances stimulated mitochondrial Ca2+ uptake in RBL cells as well as in HEK293 and N2a cells, which are commonly used to model neurons. We find that helix 1/2 perturbations nullify this enhancing effect, consistent with the N-terminal membrane binding portion of a-syn facilitating the increase in mitochondrial Ca2+ uptake. Current results indicate that the source of Ca2+ for mitochondrial uptake is mainly from the endoplasmic reticulum (ER). We employ structured illumination microscopy to examine the relationship between the increase in mitochondrial Ca2+ uptake and the contacts between ER and mitochondria. We are now investigating a novel inhibitory effect of a-syn on recovery from mitochondrial stress caused by PD-inducing toxins. Our results highlight the significance of specific structural features of a-syn in regulating vesicle release and modulating mitochondrial Ca2+ uptake, and they point to a novel pathological function for a-syn under mitochondrial stress related to PD.
Ramezani, M.†, M.M. Wilkes†, T. Das, D. Holowka, D. Eliezer*, B. Baird*: Regulation of exocytosis and mitochondrial relocalization by Alpha-synuclein in a mammalian cell model. npj Parkinson’s Disease 5(1), 1-17 (2019).